Intro
0:00Organic Synthesis Strategies
0:15Goal
0:16Strategy
0:29
Example of a RetroSynthesis
1:30Finding Starting Materials for Target Molecule
1:31Synthesis Using Starting Materials
4:56
Synthesis of Alcohols by Functional Group Interconversion (FGI)
6:00Synthesis of Alcohols by Functional Group Interconversion Overview
6:01
Alcohols by Reduction
7:43Ketone to Alcohols
7:45Aldehyde to Alcohols
8:26Carboxylic Acid Derivative to Alcohols
8:36
Alcohols by Hydration of Alkenes
9:28Hydration of Alkenes Using H₃O⁺
9:29Oxymercuration-Demercuration
10:35Hydroboration Oxidation
11:02
Alcohols by Substitution
11:42Primary Alkyl Halide to Alcohols Using NaOH
11:43Secondary Alkyl Halide to Alcohols Using Sodium Acetate
13:07Tertiary Alkyl Halide to Alcohols Using H₂O
15:08
Synthesis of Alcohols by Forming a New C-C Bond
15:47Recall: Alcohol & RMgBr
15:48Retrosynthesis
17:28
Other Alcohol Disconnections
19:46
19:47Synthesis Using PhMGgBr: Example 2
23:05
Synthesis of Alkyl Halides
26:06Synthesis of Alkyl Halides Overview
26:07
Synthesis of Alkyl Halides by Free Radical Halogenation
27:04Synthesis of Alkyl Halides by Free Radical Halogenation
27:05
Synthesis of Alkyl Halides by Substitution
29:06Alcohol to Alkyl Halides Using HBr or HCl
29:07Alcohol to Alkyl Halides Using SOCl₂
30:57Alcohol to Alkyl Halides Using PBr₃ and Using P, I₂
31:03
Synthesis of Alkyl Halides by Addition
32:02Alkene to Alkyl Halides Using HBr
32:03Alkene to Alkyl Halides Using HBr & ROOR (Peroxides)
32:35
Example: Synthesis of Alkyl Halide
34:18Example: Synthesis of Alkyl Halide
34:19
Synthesis of Ethers
39:25Synthesis of Ethers
39:26
Example: Synthesis of an Ether
41:12Synthesize TBME (t-butyl methyl ether) from Alcohol Starting Materials
41:13
Synthesis of Amines
46:05Synthesis of Amines
46:06
Gabriel Synthesis of Amines
47:57Gabriel Synthesis of Amines
47:58
Amines by SN2 with Azide Nu:
49:50Amines by SN2 with Azide Nu:
49:51
Amines by SN2 with Cyanide Nu:
50:31Amines by SN2 with Cyanide Nu:
50:32
Amines by Reduction of Amides
51:30Amines by Reduction of Amides
51:31
Reductive Amination of Ketones/Aldehydes
52:42Reductive Amination of Ketones/Aldehydes
52:43
Example : Synthesis of an Amine
53:47Example 1: Synthesis of an Amine
53:48Example 2: Synthesis of an Amine
56:16
Synthesis of Alkenes
58:20Synthesis of Alkenes Overview
58:21
Synthesis of Alkenes by Elimination
59:04Synthesis of Alkenes by Elimination Using NaOH & Heat
59:05Synthesis of Alkenes by Elimination Using H₂SO₄ & Heat
59:57
Synthesis of Alkenes by Reduction
1:02:05Alkyne to Cis Alkene
1:02:06Alkyne to Trans Alkene
1:02:56
Synthesis of Alkenes by Wittig Reaction
1:03:46Synthesis of Alkenes by Wittig Reaction
1:03:47Retrosynthesis of an Alkene
1:05:35
Example: Synthesis of an Alkene
1:06:57Example: Synthesis of an Alkene
1:06:58Making a Wittig Reagent
1:10:31
Synthesis of Alkynes
1:13:09Synthesis of Alkynes
1:13:10
Synthesis of Alkynes by Elimination (FGI)
1:13:42First Step: Bromination of Alkene
1:13:43Second Step: KOH Heat
1:14:22
Synthesis of Alkynes by Alkylation
1:15:02Synthesis of Alkynes by Alkylation
1:15:03Retrosynthesis of an Alkyne
1:16:18
Example: Synthesis of an Alkyne
1:17:40Example: Synthesis of an Alkyne
1:17:41
Synthesis of Alkanes
1:20:52Synthesis of Alkanes
1:20:53
Synthesis of Aldehydes & Ketones
1:21:38Oxidation of Alcohol Using PCC or Swern
1:21:39Oxidation of Alkene Using 1) O₃, 2)Zn
1:22:42Reduction of Acid Chloride & Nitrile Using DiBAL-H
1:23:25Hydration of Alkynes
1:24:55Synthesis of Ketones by Acyl Substitution
1:26:12Reaction with R'₂CuLi
1:26:13Reaction with R'MgBr
1:27:13
Synthesis of Aldehydes & Ketones by α-Alkylation
1:28:00Synthesis of Aldehydes & Ketones by α-Alkylation
1:28:01Retrosynthesis of a Ketone
1:30:10
Acetoacetate Ester Synthesis of Ketones
1:31:05Acetoacetate Ester Synthesis of Ketones: Step 1
1:31:06Acetoacetate Ester Synthesis of Ketones: Step 2
1:32:13Acetoacetate Ester Synthesis of Ketones: Step 3
1:32:50
Example: Synthesis of a Ketone
1:34:11Example: Synthesis of a Ketone
1:34:12
Synthesis of Carboxylic Acids
1:37:15Synthesis of Carboxylic Acids
1:37:16
Example: Synthesis of a Carboxylic Acid
1:37:59Example: Synthesis of a Carboxylic Acid (Option 1)
1:38:00Example: Synthesis of a Carboxylic Acid (Option 2)
1:40:51
Malonic Ester Synthesis of Carboxylic Acid
1:42:34Malonic Ester Synthesis of Carboxylic Acid: Step 1
1:42:35Malonic Ester Synthesis of Carboxylic Acid: Step 2
1:43:36Malonic Ester Synthesis of Carboxylic Acid: Step 3
1:44:01
Example: Synthesis of a Carboxylic Acid
1:44:53Example: Synthesis of a Carboxylic Acid
1:44:54
Synthesis of Carboxylic Acid Derivatives
1:48:05Synthesis of Carboxylic Acid Derivatives
1:48:06
Alternate Ester Synthesis
1:48:58Using Fischer Esterification
1:48:59Using SN2 Reaction
1:50:18Using Diazomethane
1:50:56Using 1) LDA, 2) R'-X
1:52:15
Practice: Synthesis of an Alkyl Chloride
1:53:11Practice: Synthesis of an Alkyl Chloride
1:53:12
Patterns of Functional Groups in Target Molecules
1:59:53Recall: Aldol Reaction
1:59:54β-hydroxy Ketone Target Molecule
2:01:12α,β-unsaturated Ketone Target Molecule
2:02:20
Patterns of Functional Groups in Target Molecules
2:03:15Recall: Michael Reaction
2:03:16Retrosynthesis: 1,5-dicarbonyl Target Molecule
2:04:07
Patterns of Functional Groups in Target Molecules
2:06:38Recall: Claisen Condensation
2:06:39Retrosynthesis: β-ketoester Target Molecule
2:07:30
2-Group Target Molecule Summary
2:09:032-Group Target Molecule Summary
2:09:04
Example: Synthesis of Epoxy Ketone
2:11:19Synthesize the Following Target Molecule from Cyclohexanone: Part 1 - Retrosynthesis
2:11:20Synthesize the Following Target Molecule from Cyclohexanone: Part 2 - Synthesis
2:14:10
Example: Synthesis of a Diketone
2:16:57Synthesis of a Diketone: Step 1 - Retrosynthesis
2:16:58Synthesis of a Diketone: Step 2 - Synthesis
2:18:51











































 Answer Engine
Answer Engine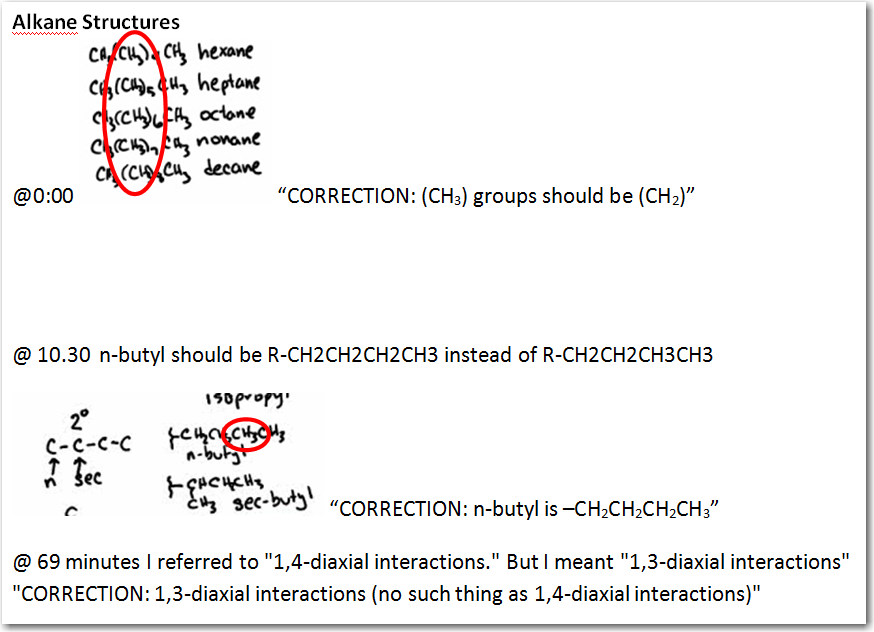



















1 answer
Sun May 19, 2024 2:31 AM
Post by Christina Whitmore on May 18, 2024
Hello! I am really enjoying your lectures! Where did you buy the really big molecular model kit? I teach at a community college and would love to have big models to work with.
1 answer
Thu Oct 24, 2019 9:31 PM
Post by Euichul Jung on October 16, 2019
I was studying the practice section and could you re-post the answer for the last question "Draw 3 constitutional isomers for C7H16."? It shows the previous answer.
1 answer
Tue Nov 13, 2018 12:51 PM
Post by Angela Mercado on October 17, 2018
Hi professor,
I was wondering @14.42 why we are not labeling the compound the opposite way? I thought alkyl halides take priority over regular alkyl substituents when numbering the longest carbon chain? Would the compound not be 2,3-dichloro-7,7-dimethyloctane?
1 answer
Sat Jan 13, 2018 6:41 PM
Post by Jade Huynh on November 22, 2017
Hi Professor Starkey! I was wondering if we were to have a nitrogen and/or phosphorus in our molecular formula, would we just subtract one hydrogen per nitrogen and/or phosphorus from the alkane formula?
For example, if we were asked to find the DU of the the following formula C9H11OP and I wanted to compare it to the alkane formula, would the alkane formula be would C9H19?
1 answer
Tue Oct 10, 2017 1:32 AM
Post by Maryam Fayyazi on October 5, 2017
How to distinguish between cis,trans isomers and eclipse,staggerd?
1 answer
Fri Feb 3, 2017 9:57 PM
Post by FALIKOU DUKULY on January 16, 2017
hi Dr.Starkey, how can you have CH3(CH3)4CH3 as pentane? isn't is supposed to be CH3(CH2)4CH3? if not how does CH3(CH3)4CH3 bond to each other?
2 answers
Last reply by: omeed tarzi
Thu Oct 6, 2016 2:01 PM
Post by omeed tarzi on October 6, 2016
is numbering right for 6,7-dichloro-2,2-dimthyl ......
?????? why we not start numbering from cl ????? plz explain
1 answer
Mon Mar 16, 2015 11:33 PM
Post by Saadman Elman on March 15, 2015
Hi, Professor, Starkey. As usual great lecture. At 68:30 min When you were explaining which side of cyclohexane is more stable. You said the one in the left is less stable and the reason is Ch3 in the axial side has a steric of 1-4 Diaxial interaction. I think you meant to say 1-3 diaxial interaction and Not 1-4 Diaxial interaction. Am i correct? Or i am having misunderstanding. I think that's what my professor said.
1 answer
Sat Feb 21, 2015 11:22 PM
Post by amanda Cormier on February 19, 2015
How did you get to be so awesome? You're saving my life in Ochem!
1 answer
Fri Dec 5, 2014 1:19 AM
Post by Camille Fraser on December 3, 2014
In the very last question in minuet 69 of Cyclohexane Conformation example do you first always have to draw the original structure and then put it in the chair? Also is that a methyl group attached to the t-butyl in the cyclohexane model...at the base?
1 answer
Fri Dec 5, 2014 1:22 AM
Post by Camille Fraser on December 3, 2014
Please when writing on the board closer to the bottom make sure WE can all see the problem. When explaining the Alkanes at the end I could not see the other Chloro group in the octane molecule and the other subs were not clear closer to the front end of the structure.
1 answer
Thu Aug 7, 2014 8:47 PM
Post by David Gonzalez on August 5, 2014
I know this is slightly off topic, but is a fatty acid considered an alkane (since it's made up of hydrocarbons)? Thank you professor Starkey.
1 answer
Sat Jul 19, 2014 10:23 PM
Post by David Gonzalez on July 17, 2014
What if there are two groups coming from the same carbon? How would you name that? Thanks Professor Starkey.
1 answer
Mon Jun 23, 2014 1:22 PM
Post by Neil Choudhry on June 22, 2014
For the degrees of unsaturation, what do you do if there are Nitrogen atoms? For example, C3H9N
1 answer
Sat May 10, 2014 12:05 AM
Post by somia abdelgawad on May 7, 2014
on 69 minute is 1,4 diaxial or 1, 3 diaxial interaction.
2 answers
Last reply by: brandon oneal
Sat Nov 23, 2013 12:36 PM
Post by brandon oneal on November 15, 2013
Will you have lectures for organic chemistry II?
1 answer
Sun Sep 29, 2013 1:55 PM
Post by Shawn Ng on September 28, 2013
Hi Dr. Starkey,
At 10.30, shouldn't n-butyl be R-CH2CH2CH2CH3 instead of R-CH2CH2CH3CH3?
1 answer
Sun Sep 15, 2013 1:45 PM
Post by Kingsley Lunga on September 14, 2013
I am feeling extremely disappointed right now, I just purchased a subscription yester and I am not able to watch a video consistently for 10 minutes without it freezing on me. Also, it wont let me go to a specific topic that i'd like to watch, if you click on any sub topic, it takes you to the Main topic and this is very frustration, plus it wont even let you fast forward....please can someone offer some suggestions for me, all my excitement about educator.com is doing down.
1 answer
Sun Sep 15, 2013 3:23 PM
Post by Kingsley Lunga on September 14, 2013
I am using high speed internet with a very good laptop but the lecture keeps freezing after every 5 minutes, is anyone experiencing this?
1 answer
Tue Sep 10, 2013 4:34 PM
Post by Fadel Hanoun on September 9, 2013
Thank you for breaking this complicated subject so well. Is it possible to get a copy of the lecture slides in higher resolution?
1 answer
Mon Jul 22, 2013 12:00 AM
Post by Fadel Hanoun on July 21, 2013
I am wondering how come Energy of B > C when the methyl groups are closer apart in C than in B.
1 answer
Thu Jul 11, 2013 3:40 PM
Post by noelle edejer on July 11, 2013
i cant see the bottom and the writing is not clear
1 answer
Last reply by: Nawaphan Jedjomnongkit
Mon Apr 29, 2013 3:11 AM
Post by Nawaphan Jedjomnongkit on April 29, 2013
from second slide on nomenclature of alkanes Why CH3CH2- is propyl not ethyl?
1 answer
Fri Mar 1, 2013 11:22 PM
Post by James Bond on March 1, 2013
On 8:12, what if there were 2 methyl groups attached at carbon 2. What would the name be then?
1 answer
Tue Feb 12, 2013 10:45 AM
Post by SINA AZADI on February 11, 2013
Thank you so much Dr. Starkey. You are the most helpful teacher I ever had in my chemistry courses. Barely Understood some concepts.
1 answer
Mon Oct 15, 2012 9:55 PM
Post by noor almakabi on October 14, 2012
Hi Starkey,
Can we say for 2-methylpropane that it has 4 eclipsed conformation at 4 different degrees (0â°,360â°,120â°,240â°) and 2 staggered conformation at 3 different degrees (60â°,300â°,180â°)?
1 answer
Fri Oct 12, 2012 8:36 PM
Post by José Menéndez on October 12, 2012
hey, how do I know it's a 60 degrees rotation or 120 etc?
1 answer
Last reply by: José Menéndez
Fri Oct 12, 2012 1:00 PM
Post by Paula Hoggard on October 11, 2012
How do I get to newman right away? I want to skip the beginning and I can't click on what I want!
1 answer
Tue Oct 2, 2012 11:24 PM
Post by Ty Smith on October 1, 2012
You are an excellent teacher. Thank you for doing these.
1 answer
Wed Jan 4, 2012 10:53 PM
Post by Lilian Comparini on January 2, 2012
You are amazing, I finally get concepts that until you explained them seemed complicated. thank you! I am more confident about taking my DAT thanks to you.
1 answer
Thu Oct 20, 2011 11:45 PM
Post by SHADLIN MOTARASSED on October 15, 2011
Is Example 3 when discussing DU Benzene? I think it is Hexene???
1 answer
Sun Sep 25, 2011 7:28 PM
Post by valerie phan on September 18, 2011
I really like this video and the tools you used! I wish there are more professors like you. Thank you so much. I just wanted to say that because you made ochem seem less scary compare to the rumors. =[
1 answer
Sat Jul 30, 2011 12:07 AM
Post by Daniela Valencia on July 2, 2011
Dr. Starkey
I love how you teach, your videos have helped me a lot with my studying, I will be taking my PCAT test next month. Thank you!!! You're a great teacher!
1 answer
Sat Jul 30, 2011 12:07 AM
Post by chen yakar on June 29, 2011
This is great!!
1 answer
Sat Jul 30, 2011 12:08 AM
Post by Priscilla Seabourn on June 27, 2011
Dr. Starkey, you have been extremely helpful. I am taking my MCAT soon, and you have helped tremendously! Thank you.
0 answers
Post by Billy Jay on April 12, 2011
Awesome. Can't wait to see those. Any idea if they'll be uploaded before June? If you're unsure, it's fine. I was just curious because my MCAT is scheduled for that date. Thank you.
3 answers
Mon Jul 8, 2013 11:13 PM
Post by Billy Jabbar on March 15, 2011
Hi Dr. Starkey,
For the chair conformation of cyclohexane - you mention that there are "1,4-diaxial interactions." I'm thinking you meant "1,3-diaxial interactions" instead, am I right? :)
Also, well done on the videos. They've been extremely helpful so far. I enjoy your enthusiasm (it's very contagious) and it's made this learning experience very enjoyable :)
One more question though: I noticed a few topics are left out such as Spectroscopy. Any chance those will be included in the future?
Thank you.
0 answers
Post by Professor Starkey on February 4, 2011
At 13:48, an extra example was drawn on screen, but the bottom constituent was cut off by the navigation bar. Would be nice if the navigation bar could be hidden with a button for such occasions. Another idea would be to just have the navigation bar not in the window at all, that way it would never even need to be hidden.