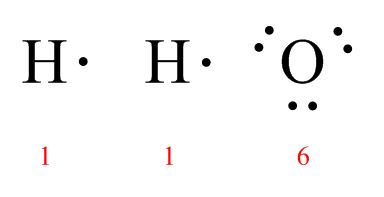
It may look like the periodic table is coming down with the chicken pox, but Lewis Dot Structures are a core part of chemistry. They bring together several important concepts about how atoms and molecules work and help you visualize important compounds, so it’s worth getting these right!
Molecular Formula
You’re probably pretty familiar with compounds like CO2 and H2O – Carbon Dioxide and water. But what do they actually look like? How are the different atoms bonded together? And how do you figure out what order to put the atoms in?
The Secret: Valence Electrons
Atoms bond together to form molecules for one reason: stability. Most elements need eight electrons in their outer shell to become stable and non-reactive, and they will form bonds with other elements in order to get the electrons they need.
You can tell how many outer or “valence” electrons an atom starts with by looking at the column it sits in on the periodic table. Carbon has four, Oxygen has six, and Hydrogen has one. When forming molecules, atoms generally join together using single, double, or triple bonds—whatever they need to do to end up with eight electrons. Extra, non-bonding electrons are called “lone pairs.”
Drawing the Structure
Follow these five simple steps and you’ll be able to figure out the structure of almost any compound. We’ll start with two simple ones: CO2 and H2O.
Step 1: Start by identifying the central atom, and draw out/count up all the valence electrons you have to work with.
The central atom is usually the one that appears just once, and it’s usually not hydrogen. If there’s a carbon present, that’s a good bet. If more than one element appears multiple times, choose the least electronegative atom—the one that sits the furthest to the left on the periodic table. This element will be your central building block.
H2O: O is the central atom because it only appears once. There are 8 total electrons to work with.
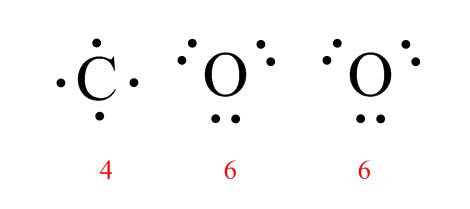
CO2: In this case, C will be the central atom because it only appears once. This molecule has 16 valence electrons to work with.
If you’re working with a polyatomic ion, remember that electrons are negatively charged. If the ion has a positive charge, subtract one electron; if there’s a negative charge, add an extra electron to the count. You’ll figure out where to put it later when you make sure each element has its octet.
Step 2: Arrange the atoms around the center one and connect them with single bonds to start.
Leave the lone pair electrons where they are for the moment:
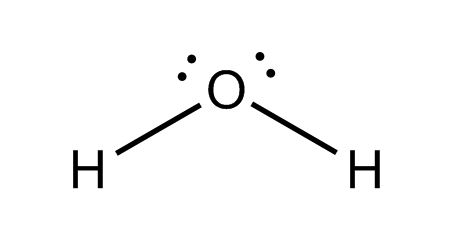
H2O: O is the central atom
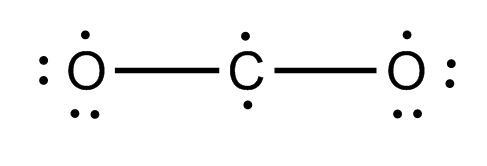
CO2: C is the central atom
Step 3: Count to see if each atom has a complete octet.
There are a few exceptions to the octet rule, and it’s important to memorize them because chemistry teachers love to use them in problems to see if you’ve been paying attention. Hydrogen only needs two outer electrons; Beryllium (Be) only needs 4; Boron (B) and Aluminum (Al) only need 6, and Phosphorus (P) and Sulfur (S) can have more than eight, known as an expanded octet.
Remember that single bonds have two electrons, double bonds have four, and triple bonds have six.
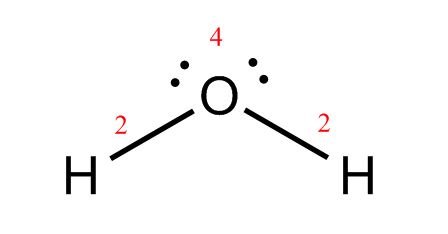
H2O: The O has two lone pairs plus two single bonds attached to it for a total of 8 electrons. Its octet is complete! Hydrogen only needs two valence electrons, and each one has a single bond attached, so both hydrogens are complete as well. This diagram is all finished, except for a double check!
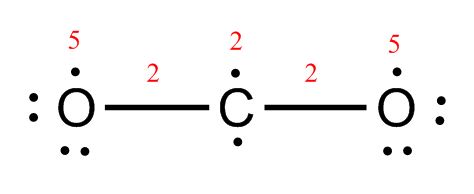
CO2: Each oxygen has 5 electrons, plus the 2 in the single bond, for a total of 7 – not quite enough to complete the octet. Carbon has 2 electrons plus 2 single bonds (2×2 electrons) for a total of 6 electrons—also not enough to complete the octet.
Step 4: Use multiple bonds to complete the octet (if needed).
In this case, H2O was complete using only single bonds, but CO2 didn’t quite work. Try using double bonds to make sure every atom gets its complete octet:
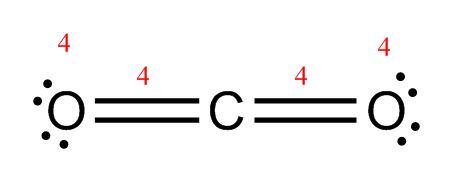
CO2: Now, each oxygen has two lone pairs for a total of 4 electrons and a double bond for 4 more electrons. That adds up to 8! Carbon has two double bonds, which means it also has 8 electrons. You’re done!
Step 5: Count up the electrons to double check the structure.
Make sure you end up with the same number of electrons you started with. H2O started with 8. Two lone pairs plus two bonds equals eight, so that one passes the test. CO2 started with 16. The dot diagram shows four lone pairs (4×2 = 8 electrons) plus two double bonds (2×4 = 8 electrons) for a total of 16. It also passes the test!
Now that you can draw basic Lewis Dot Diagrams and know where all the bonds go, you can do things like figure out the shapes of molecules and label resonance structures. These diagrams are the building blocks of chemistry—and all of the important molecules that make up the world around us—so stick with it. You’ll be well rewarded!
More examples and a slightly different method
[box type=”success” align=”” class=”” width=””]If you follow these five simple steps, you’ll be able to figure out the structure of almost any compound. For more examples and a slightly different method, check out the Bonding & Lewis Structure lesson in our AP Chemistry course or the Lewis Structure & Resonance lesson in our Organic Chemistry class.[/box]