Intro
0:00Organic Synthesis Strategies
0:15Goal
0:16Strategy
0:29
Example of a RetroSynthesis
1:30Finding Starting Materials for Target Molecule
1:31Synthesis Using Starting Materials
4:56
Synthesis of Alcohols by Functional Group Interconversion (FGI)
6:00Synthesis of Alcohols by Functional Group Interconversion Overview
6:01
Alcohols by Reduction
7:43Ketone to Alcohols
7:45Aldehyde to Alcohols
8:26Carboxylic Acid Derivative to Alcohols
8:36
Alcohols by Hydration of Alkenes
9:28Hydration of Alkenes Using H₃O⁺
9:29Oxymercuration-Demercuration
10:35Hydroboration Oxidation
11:02
Alcohols by Substitution
11:42Primary Alkyl Halide to Alcohols Using NaOH
11:43Secondary Alkyl Halide to Alcohols Using Sodium Acetate
13:07Tertiary Alkyl Halide to Alcohols Using H₂O
15:08
Synthesis of Alcohols by Forming a New C-C Bond
15:47Recall: Alcohol & RMgBr
15:48Retrosynthesis
17:28
Other Alcohol Disconnections
19:46
19:47Synthesis Using PhMGgBr: Example 2
23:05
Synthesis of Alkyl Halides
26:06Synthesis of Alkyl Halides Overview
26:07
Synthesis of Alkyl Halides by Free Radical Halogenation
27:04Synthesis of Alkyl Halides by Free Radical Halogenation
27:05
Synthesis of Alkyl Halides by Substitution
29:06Alcohol to Alkyl Halides Using HBr or HCl
29:07Alcohol to Alkyl Halides Using SOCl₂
30:57Alcohol to Alkyl Halides Using PBr₃ and Using P, I₂
31:03
Synthesis of Alkyl Halides by Addition
32:02Alkene to Alkyl Halides Using HBr
32:03Alkene to Alkyl Halides Using HBr & ROOR (Peroxides)
32:35
Example: Synthesis of Alkyl Halide
34:18Example: Synthesis of Alkyl Halide
34:19
Synthesis of Ethers
39:25Synthesis of Ethers
39:26
Example: Synthesis of an Ether
41:12Synthesize TBME (t-butyl methyl ether) from Alcohol Starting Materials
41:13
Synthesis of Amines
46:05Synthesis of Amines
46:06
Gabriel Synthesis of Amines
47:57Gabriel Synthesis of Amines
47:58
Amines by SN2 with Azide Nu:
49:50Amines by SN2 with Azide Nu:
49:51
Amines by SN2 with Cyanide Nu:
50:31Amines by SN2 with Cyanide Nu:
50:32
Amines by Reduction of Amides
51:30Amines by Reduction of Amides
51:31
Reductive Amination of Ketones/Aldehydes
52:42Reductive Amination of Ketones/Aldehydes
52:43
Example : Synthesis of an Amine
53:47Example 1: Synthesis of an Amine
53:48Example 2: Synthesis of an Amine
56:16
Synthesis of Alkenes
58:20Synthesis of Alkenes Overview
58:21
Synthesis of Alkenes by Elimination
59:04Synthesis of Alkenes by Elimination Using NaOH & Heat
59:05Synthesis of Alkenes by Elimination Using H₂SO₄ & Heat
59:57
Synthesis of Alkenes by Reduction
1:02:05Alkyne to Cis Alkene
1:02:06Alkyne to Trans Alkene
1:02:56
Synthesis of Alkenes by Wittig Reaction
1:03:46Synthesis of Alkenes by Wittig Reaction
1:03:47Retrosynthesis of an Alkene
1:05:35
Example: Synthesis of an Alkene
1:06:57Example: Synthesis of an Alkene
1:06:58Making a Wittig Reagent
1:10:31
Synthesis of Alkynes
1:13:09Synthesis of Alkynes
1:13:10
Synthesis of Alkynes by Elimination (FGI)
1:13:42First Step: Bromination of Alkene
1:13:43Second Step: KOH Heat
1:14:22
Synthesis of Alkynes by Alkylation
1:15:02Synthesis of Alkynes by Alkylation
1:15:03Retrosynthesis of an Alkyne
1:16:18
Example: Synthesis of an Alkyne
1:17:40Example: Synthesis of an Alkyne
1:17:41
Synthesis of Alkanes
1:20:52Synthesis of Alkanes
1:20:53
Synthesis of Aldehydes & Ketones
1:21:38Oxidation of Alcohol Using PCC or Swern
1:21:39Oxidation of Alkene Using 1) O₃, 2)Zn
1:22:42Reduction of Acid Chloride & Nitrile Using DiBAL-H
1:23:25Hydration of Alkynes
1:24:55Synthesis of Ketones by Acyl Substitution
1:26:12Reaction with R'₂CuLi
1:26:13Reaction with R'MgBr
1:27:13
Synthesis of Aldehydes & Ketones by α-Alkylation
1:28:00Synthesis of Aldehydes & Ketones by α-Alkylation
1:28:01Retrosynthesis of a Ketone
1:30:10
Acetoacetate Ester Synthesis of Ketones
1:31:05Acetoacetate Ester Synthesis of Ketones: Step 1
1:31:06Acetoacetate Ester Synthesis of Ketones: Step 2
1:32:13Acetoacetate Ester Synthesis of Ketones: Step 3
1:32:50
Example: Synthesis of a Ketone
1:34:11Example: Synthesis of a Ketone
1:34:12
Synthesis of Carboxylic Acids
1:37:15Synthesis of Carboxylic Acids
1:37:16
Example: Synthesis of a Carboxylic Acid
1:37:59Example: Synthesis of a Carboxylic Acid (Option 1)
1:38:00Example: Synthesis of a Carboxylic Acid (Option 2)
1:40:51
Malonic Ester Synthesis of Carboxylic Acid
1:42:34Malonic Ester Synthesis of Carboxylic Acid: Step 1
1:42:35Malonic Ester Synthesis of Carboxylic Acid: Step 2
1:43:36Malonic Ester Synthesis of Carboxylic Acid: Step 3
1:44:01
Example: Synthesis of a Carboxylic Acid
1:44:53Example: Synthesis of a Carboxylic Acid
1:44:54
Synthesis of Carboxylic Acid Derivatives
1:48:05Synthesis of Carboxylic Acid Derivatives
1:48:06
Alternate Ester Synthesis
1:48:58Using Fischer Esterification
1:48:59Using SN2 Reaction
1:50:18Using Diazomethane
1:50:56Using 1) LDA, 2) R'-X
1:52:15
Practice: Synthesis of an Alkyl Chloride
1:53:11Practice: Synthesis of an Alkyl Chloride
1:53:12
Patterns of Functional Groups in Target Molecules
1:59:53Recall: Aldol Reaction
1:59:54β-hydroxy Ketone Target Molecule
2:01:12α,β-unsaturated Ketone Target Molecule
2:02:20
Patterns of Functional Groups in Target Molecules
2:03:15Recall: Michael Reaction
2:03:16Retrosynthesis: 1,5-dicarbonyl Target Molecule
2:04:07
Patterns of Functional Groups in Target Molecules
2:06:38Recall: Claisen Condensation
2:06:39Retrosynthesis: β-ketoester Target Molecule
2:07:30
2-Group Target Molecule Summary
2:09:032-Group Target Molecule Summary
2:09:04
Example: Synthesis of Epoxy Ketone
2:11:19Synthesize the Following Target Molecule from Cyclohexanone: Part 1 - Retrosynthesis
2:11:20Synthesize the Following Target Molecule from Cyclohexanone: Part 2 - Synthesis
2:14:10
Example: Synthesis of a Diketone
2:16:57Synthesis of a Diketone: Step 1 - Retrosynthesis
2:16:58Synthesis of a Diketone: Step 2 - Synthesis
2:18:51











































 Answer Engine
Answer Engine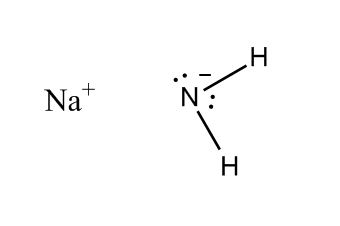









1 answer
Mon Jun 15, 2020 3:36 PM
Post by byronglc on May 15, 2020
Dear teacher,
what does mean this resalted and dash bonds in cholesterol? min 38
1 answer
Fri Aug 2, 2019 11:50 AM
Post by Said Sabir on August 2, 2019
Professoer, I need to understand more about the orbitals and how the electrons are held
1 answer
Wed Sep 12, 2018 5:07 PM
Post by Mohamed Abdulle on September 2, 2018
Hi, what you mean by dipole dipole moment when you were talking about the polarity of molecule in a linear form.
1 answer
Fri Jan 26, 2018 12:01 PM
Post by Carlins Almonor on January 23, 2018
On line drawings 4 and 6, what are the chemical names for these molecules? I am unable to find them online, although I might not have the correct symbol placement for these.
Also, for drawing 6, what happened to that first carbon?
1 answer
Tue Oct 24, 2017 8:22 PM
Post by Alain delice on October 23, 2017
Is there a better way to print the lecture notes,I'm having a lot of difficulties.Thanks
1 answer
Fri Sep 15, 2017 5:41 PM
Post by Ruben Zorrilla on September 12, 2017
Is there any way that the lecture slides could be downloaded as a 6 slides handout. I tried downloading them and they do not download in order. Having the option to print a 6 slides handout per page pdf would be very helpful. Thanks.
2 answers
Last reply by: Jane Fritchley
Mon Jul 31, 2017 9:08 PM
Post by Jane Fritchley on July 27, 2017
Is there a textbook that accompanies your teaching? Or should I purchase the Wade or Klein texts that are listed as related materials? Thank you, I really enjoy your teaching.
1 answer
Fri Mar 31, 2017 10:15 PM
Post by Maria Mazos on March 25, 2017
38:00 min- in the structure of DNA, OH have to be on carbon 3' but it on carbon 5'.. it should be reversed i think..
1 answer
Fri Feb 3, 2017 9:56 PM
Post by Anastasia Asante on January 10, 2017
does carbon and boron want to be like the noble has, i though carbon can be attached to just 4 atoms. ??
1 answer
Thu Nov 3, 2016 11:57 AM
Post by Firebird wang on November 2, 2016
Professor, I know that AP Statistics is not your subject, but I just wonder if you are able to watch the two videos which called "Practice Test 2013 AP Statistics" and "Practice Test 2014 AP Statistics" in the AP Statistics content? Both videos showing network error, I dont know why. I already tried in different computers already.
1 answer
Fri May 13, 2016 11:20 AM
Post by Mohamed E Sowaileh on May 11, 2016
Thank you for the immediate response. I am very grateful to you for the invaluable help you gave me. May God bless you.
3 answers
Mon May 9, 2016 3:37 PM
Post by Mohamed E Sowaileh on May 8, 2016
Dr. Laurie Starkey,
I hope you are doing very well,
Someone has never taken a deep and comprehensive general chemistry course, do you recommend him to take G. chemistry courses to have a solid understanding in chemistry, because I guess organic chemistry then would be a lot more easier than if I didn't take G. chemistry. am I correct? or do you think that the quick review you made is quite sufficient? I appreciate your help.
Thank you.
2 answers
Last reply by: Apolonia Gardner
Wed Dec 2, 2015 12:40 AM
Post by Apolonia Gardner on November 24, 2015
Hello,
I am a high school senior about to send off my applications for college. I am stuck on one thing – my intended major. Biology and chemistry have been my favorite courses throughout high school, and I would like to get a college degree that will enable me to perform research with viruses. My lifetime goal is to find a cure for a disease. From your experience, what undergraduate major should I shoot for? Biochemistry? Microbiology? Molecular Biology? Immunology? Chemical Biology? Organic Chemistry? Pharmaceutical Science? Any guidance is appreciated.
5 answers
Mon Jun 8, 2015 1:26 AM
Post by Jinbin Chen on June 5, 2015
Hi, Dr. Starkey!
I have watched some of your lectures to prepare for the national chemistry olympiad exam, and even though the pace of these lectures are kind of fast, I really like you explanations and passion for organic chemistry.
Right now, I am planning on doing organic chemistry in my freshman year of college with the help of AP credits. Do you have any suggestions on things that I should do in the summer to adequately prepare for organic chemistry?
1 answer
Wed Jan 7, 2015 7:44 PM
Post by Galymzhan Tuleushov on January 6, 2015
wqs
1 answer
Tue Dec 16, 2014 1:27 PM
Post by Rene Whitaker on December 15, 2014
I don't have a question, I just want to thank you. I relied on rewatching your videos to prepare for my cumulative final in Orgo I, which was a national standardized test. My professor said 50% is the average score on the test and I got an 84%. With the exception of synthesis, which will forever be my nemesis, I find your lectures to be extremely understandable and helpful. THANK YOU, THANK YOU, THANK YOU!!! Now, you just have to get me through Orgo II.
1 answer
Mon Jul 21, 2014 10:22 PM
Post by Saria Abbas on July 21, 2014
I have a couple of past papers but no answers to them, some are visual mechanisms, is there a way I could send you questions and/workings out for feedback?
1 answer
Tue Feb 4, 2014 8:56 PM
Post by Habib Awes on February 3, 2014
1. which are the properties of ionic and covalent substances? illustrate by reffering to: Sodiaum chloride, diamond and methene.
2. the substances which is ionic, please detail your resoning.
3. now the chosen covalent substance, may you detail your reasoning.
1 answer
Fri Dec 6, 2013 6:22 PM
Post by robina saeed on December 6, 2013
Hello Professor,
Just a quick question. I have never had Organic Chemistry and I am planning on using this course preparing for the MCAT. Good idea? take care
1 answer
Sun Dec 1, 2013 1:49 PM
Post by matatio manoah on November 30, 2013
Just wondering which subject is more based on energy chemistry or physics
1 answer
Thu Nov 28, 2013 7:14 PM
Post by Jennifer Akiga on November 28, 2013
I've studied up until Alcohols! You're an awesome teacher and explain well. I just wish I could fastworward the slides, they take ages. Helped me a lot in my test.
2 answers
Last reply by: robina saeed
Fri Oct 18, 2013 1:14 PM
Post by robina saeed on October 16, 2013
Is this a complete college course?
1 answer
Tue Oct 15, 2013 8:05 PM
Post by yannick Haberkorn on October 12, 2013
thank you so much for this grea
1 answer
Sun Sep 29, 2013 1:54 PM
Post by Hannah Barker on September 23, 2013
Professor Starkey, I am taking Organic Chemistry online this semester and I stumbled across these lectures and I bought a subscription. But my question is, can you tell me what lectures would correspond with my text book chapters? I have tried to compare the contents and sub titles of the lectures with my textbook but i havent figured it out yet. My book is "Organic Chemistry"- 9th edition by Francis A. Curey and Robert M. Giuliano. ISBN #-9780073402741
I can send the chapter titles also if you can not pull them up by that information.
Thank You!
1 answer
Thu Sep 5, 2013 1:13 PM
Post by Atreya Mohile on September 5, 2013
Do we have to remember the structures of the organic compounds, that are mentioned in the lecture, like cholesterol, etc? (Sorry for incidentally posting previous question 2 times).
1 answer
Thu Sep 5, 2013 1:05 PM
Post by Atreya Mohile on September 5, 2013
Further, in applications of SN1 and SN2 type reactions, is it required to have a grip on Chemical kinetics ? I mean, that can the questions be asked, that are based on the mixture of organic chemistry and physical chemistry in SAT subject tests or AP?
1 answer
Tue Sep 3, 2013 9:38 PM
Post by Riley Argue on September 1, 2013
Thank you.
2 answers
Tue Aug 13, 2013 7:30 PM
Post by Jeffrey Zhang on August 13, 2013
Are there any Prerequisites for this course?
1 answer
Tue Aug 13, 2013 7:31 PM
Post by Jeffrey Zhang on August 13, 2013
These Lectures are great! Are there any lecture notes or problem sets which we can work on?
1 answer
Fri Mar 1, 2013 11:19 PM
Post by James Bond on March 1, 2013
How do you know the geometry of a molecule? It's great that it can help determine if there a a net dipole or not.
2 answers
Fri Mar 1, 2013 11:26 PM
Post by Joshua Domingo on March 1, 2013
Every time I take a break, I am unable to fast forward. This is very time consuming. Is there anyway I can fast forward without waiting for a buffer?
1 answer
Thu Jan 3, 2013 10:02 AM
Post by wow love on January 3, 2013
Thanks
1 answer
Sun Nov 25, 2012 12:14 AM
Post by John Delva on November 21, 2012
why you cant fast forward to the lecture that you want????
1 answer
Fri Oct 19, 2012 10:55 AM
Post by Donna Perygin on October 17, 2012
Very good. A lot to throw out there in the first lecture. You might scare a few off, but very good.
1 answer
Sun Jun 24, 2012 10:32 AM
Post by abdualrahman m.abdualkader on June 23, 2012
My English is not that good especially in listening, so subtitles could be very helpful for me.
1 answer
Sun May 27, 2012 12:26 PM
Post by Magesh Prasanna on May 26, 2012
In the examples of polymers,styrofoam-TM,
spandex-TM, what TM represents?
I enjoyed your teaching! Thanks a lot!
0 answers
Post by Jerusalem Mulatu on April 27, 2012
You're really good with your explanations! Thank you
1 answer
Thu Oct 27, 2011 11:00 AM
Post by Alain delice on October 27, 2011
very detailed lecture, I wish my teachers would such an interest into teaching as you do; is it possible to get hand out of your lecture, maybe through a link on this website, it would very helpful.
1 answer
Wed Apr 6, 2011 11:44 PM
Post by jean philius on March 17, 2011
Does significant different mean big difference....to me it looks like hydrogen has a significant difference with carbon...meaning carbon is all the way to the right and H is to the left
1 answer
Sun Feb 6, 2011 10:05 PM
Post by Laura Diamond on December 23, 2010
HOw do you know they have two valence electrons?