Intro
0:00Organic Synthesis Strategies
0:15Goal
0:16Strategy
0:29
Example of a RetroSynthesis
1:30Finding Starting Materials for Target Molecule
1:31Synthesis Using Starting Materials
4:56
Synthesis of Alcohols by Functional Group Interconversion (FGI)
6:00Synthesis of Alcohols by Functional Group Interconversion Overview
6:01
Alcohols by Reduction
7:43Ketone to Alcohols
7:45Aldehyde to Alcohols
8:26Carboxylic Acid Derivative to Alcohols
8:36
Alcohols by Hydration of Alkenes
9:28Hydration of Alkenes Using H₃O⁺
9:29Oxymercuration-Demercuration
10:35Hydroboration Oxidation
11:02
Alcohols by Substitution
11:42Primary Alkyl Halide to Alcohols Using NaOH
11:43Secondary Alkyl Halide to Alcohols Using Sodium Acetate
13:07Tertiary Alkyl Halide to Alcohols Using H₂O
15:08
Synthesis of Alcohols by Forming a New C-C Bond
15:47Recall: Alcohol & RMgBr
15:48Retrosynthesis
17:28
Other Alcohol Disconnections
19:46
19:47Synthesis Using PhMGgBr: Example 2
23:05
Synthesis of Alkyl Halides
26:06Synthesis of Alkyl Halides Overview
26:07
Synthesis of Alkyl Halides by Free Radical Halogenation
27:04Synthesis of Alkyl Halides by Free Radical Halogenation
27:05
Synthesis of Alkyl Halides by Substitution
29:06Alcohol to Alkyl Halides Using HBr or HCl
29:07Alcohol to Alkyl Halides Using SOCl₂
30:57Alcohol to Alkyl Halides Using PBr₃ and Using P, I₂
31:03
Synthesis of Alkyl Halides by Addition
32:02Alkene to Alkyl Halides Using HBr
32:03Alkene to Alkyl Halides Using HBr & ROOR (Peroxides)
32:35
Example: Synthesis of Alkyl Halide
34:18Example: Synthesis of Alkyl Halide
34:19
Synthesis of Ethers
39:25Synthesis of Ethers
39:26
Example: Synthesis of an Ether
41:12Synthesize TBME (t-butyl methyl ether) from Alcohol Starting Materials
41:13
Synthesis of Amines
46:05Synthesis of Amines
46:06
Gabriel Synthesis of Amines
47:57Gabriel Synthesis of Amines
47:58
Amines by SN2 with Azide Nu:
49:50Amines by SN2 with Azide Nu:
49:51
Amines by SN2 with Cyanide Nu:
50:31Amines by SN2 with Cyanide Nu:
50:32
Amines by Reduction of Amides
51:30Amines by Reduction of Amides
51:31
Reductive Amination of Ketones/Aldehydes
52:42Reductive Amination of Ketones/Aldehydes
52:43
Example : Synthesis of an Amine
53:47Example 1: Synthesis of an Amine
53:48Example 2: Synthesis of an Amine
56:16
Synthesis of Alkenes
58:20Synthesis of Alkenes Overview
58:21
Synthesis of Alkenes by Elimination
59:04Synthesis of Alkenes by Elimination Using NaOH & Heat
59:05Synthesis of Alkenes by Elimination Using H₂SO₄ & Heat
59:57
Synthesis of Alkenes by Reduction
1:02:05Alkyne to Cis Alkene
1:02:06Alkyne to Trans Alkene
1:02:56
Synthesis of Alkenes by Wittig Reaction
1:03:46Synthesis of Alkenes by Wittig Reaction
1:03:47Retrosynthesis of an Alkene
1:05:35
Example: Synthesis of an Alkene
1:06:57Example: Synthesis of an Alkene
1:06:58Making a Wittig Reagent
1:10:31
Synthesis of Alkynes
1:13:09Synthesis of Alkynes
1:13:10
Synthesis of Alkynes by Elimination (FGI)
1:13:42First Step: Bromination of Alkene
1:13:43Second Step: KOH Heat
1:14:22
Synthesis of Alkynes by Alkylation
1:15:02Synthesis of Alkynes by Alkylation
1:15:03Retrosynthesis of an Alkyne
1:16:18
Example: Synthesis of an Alkyne
1:17:40Example: Synthesis of an Alkyne
1:17:41
Synthesis of Alkanes
1:20:52Synthesis of Alkanes
1:20:53
Synthesis of Aldehydes & Ketones
1:21:38Oxidation of Alcohol Using PCC or Swern
1:21:39Oxidation of Alkene Using 1) O₃, 2)Zn
1:22:42Reduction of Acid Chloride & Nitrile Using DiBAL-H
1:23:25Hydration of Alkynes
1:24:55Synthesis of Ketones by Acyl Substitution
1:26:12Reaction with R'₂CuLi
1:26:13Reaction with R'MgBr
1:27:13
Synthesis of Aldehydes & Ketones by α-Alkylation
1:28:00Synthesis of Aldehydes & Ketones by α-Alkylation
1:28:01Retrosynthesis of a Ketone
1:30:10
Acetoacetate Ester Synthesis of Ketones
1:31:05Acetoacetate Ester Synthesis of Ketones: Step 1
1:31:06Acetoacetate Ester Synthesis of Ketones: Step 2
1:32:13Acetoacetate Ester Synthesis of Ketones: Step 3
1:32:50
Example: Synthesis of a Ketone
1:34:11Example: Synthesis of a Ketone
1:34:12
Synthesis of Carboxylic Acids
1:37:15Synthesis of Carboxylic Acids
1:37:16
Example: Synthesis of a Carboxylic Acid
1:37:59Example: Synthesis of a Carboxylic Acid (Option 1)
1:38:00Example: Synthesis of a Carboxylic Acid (Option 2)
1:40:51
Malonic Ester Synthesis of Carboxylic Acid
1:42:34Malonic Ester Synthesis of Carboxylic Acid: Step 1
1:42:35Malonic Ester Synthesis of Carboxylic Acid: Step 2
1:43:36Malonic Ester Synthesis of Carboxylic Acid: Step 3
1:44:01
Example: Synthesis of a Carboxylic Acid
1:44:53Example: Synthesis of a Carboxylic Acid
1:44:54
Synthesis of Carboxylic Acid Derivatives
1:48:05Synthesis of Carboxylic Acid Derivatives
1:48:06
Alternate Ester Synthesis
1:48:58Using Fischer Esterification
1:48:59Using SN2 Reaction
1:50:18Using Diazomethane
1:50:56Using 1) LDA, 2) R'-X
1:52:15
Practice: Synthesis of an Alkyl Chloride
1:53:11Practice: Synthesis of an Alkyl Chloride
1:53:12
Patterns of Functional Groups in Target Molecules
1:59:53Recall: Aldol Reaction
1:59:54β-hydroxy Ketone Target Molecule
2:01:12α,β-unsaturated Ketone Target Molecule
2:02:20
Patterns of Functional Groups in Target Molecules
2:03:15Recall: Michael Reaction
2:03:16Retrosynthesis: 1,5-dicarbonyl Target Molecule
2:04:07
Patterns of Functional Groups in Target Molecules
2:06:38Recall: Claisen Condensation
2:06:39Retrosynthesis: β-ketoester Target Molecule
2:07:30
2-Group Target Molecule Summary
2:09:032-Group Target Molecule Summary
2:09:04
Example: Synthesis of Epoxy Ketone
2:11:19Synthesize the Following Target Molecule from Cyclohexanone: Part 1 - Retrosynthesis
2:11:20Synthesize the Following Target Molecule from Cyclohexanone: Part 2 - Synthesis
2:14:10
Example: Synthesis of a Diketone
2:16:57Synthesis of a Diketone: Step 1 - Retrosynthesis
2:16:58Synthesis of a Diketone: Step 2 - Synthesis
2:18:51










































 Answer Engine
Answer Engine
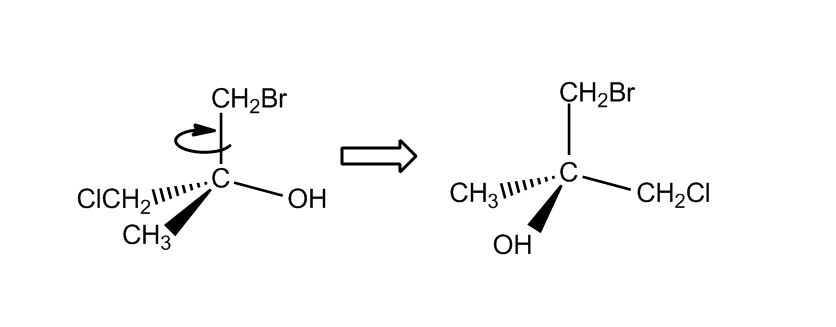
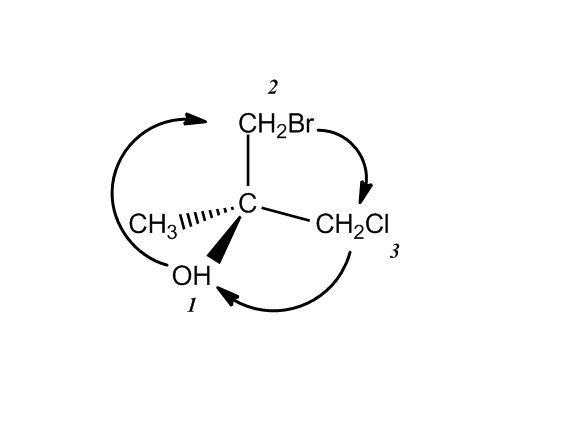
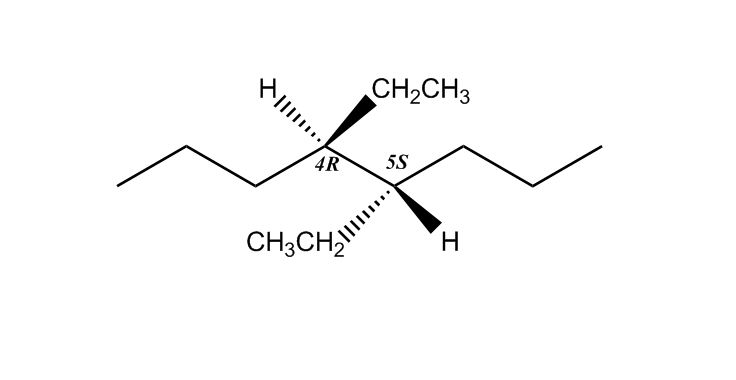
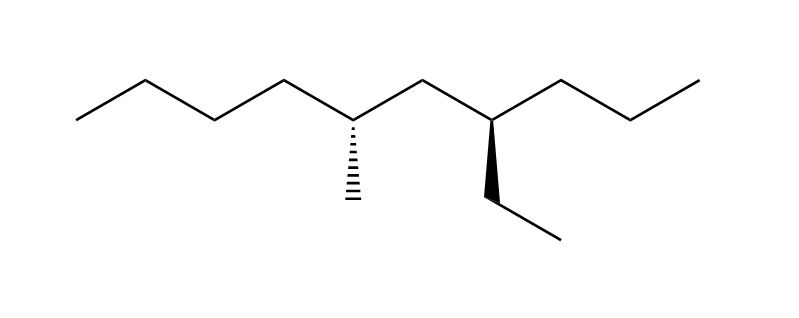









1 answer
Wed Jan 9, 2019 5:10 PM
Post by Anthony Villarama on January 9, 2019
My golly. For many years, I have not understand completely the entire topic of Stereochemisty, but today in two hours, I understood everything. You are so good that I really want to marry someone like you someday. hahahaha
1 answer
Fri Jun 22, 2018 1:39 AM
Post by Kojo Asiedu on June 16, 2018
How can I get help. The lectures are not responding. I am very frustrated because I have an exam and when I needed the site most I cant access it. It is very difficult to get help on this site
1 answer
Tue May 1, 2018 12:08 AM
Post by Arrhenius Theory on April 27, 2018
It is true that the hydrogen atom is always considered to be the lowest priority group in R and S nomenclature. Also, I would like to state whether or not if the hydrogen atom which is pointing away from the viewer follows the Cahn Ingold Prelog Convention, hence the direction of R and S nomenclature should not be reversed. Furthermore, it would suggest that it supports the Cahn Ingold Prelog convention and therefore the path in the R and S configuration should not be changed.
1 answer
Wed Dec 30, 2015 10:55 AM
Post by Shih-Kuan Chen on December 29, 2015
Hello Professor,
Your lectures are fantastic and I wanted to thank you for that.
To determine whether or not a carbon is chiral, we have to see if all 4 "groups" attached are different from one another. Does that mean, as long as the entire branch is different from each other, it is ok if the connecting atoms are the same?
Thanks
1 answer
Tue Dec 15, 2015 11:12 AM
Post by manu vats singh on December 15, 2015
which molecular model set you use
1 answer
Tue Nov 17, 2015 1:05 AM
Post by Anurag Agrawal on November 16, 2015
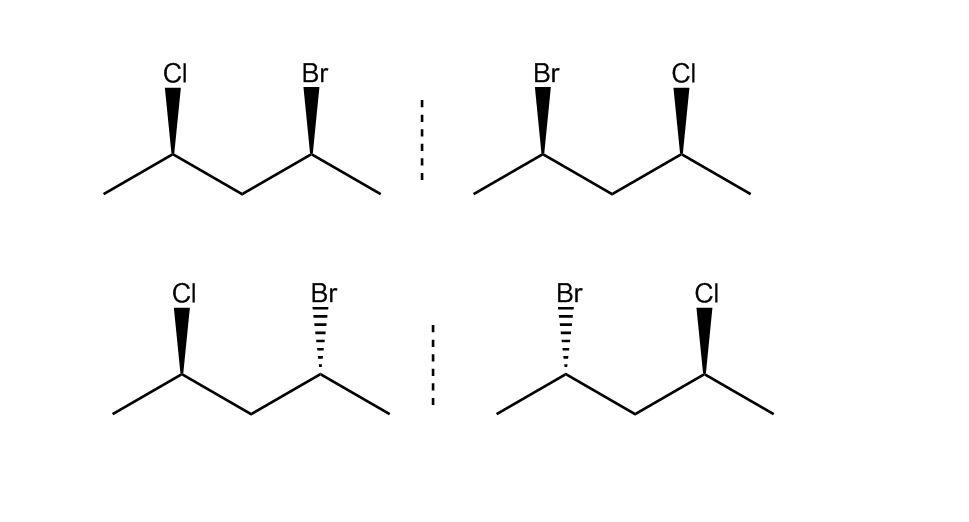
at 61:27 how do you know both bromine and chlorine are on the left for molecule C and both on the right for molecule D?
1 answer
Sun Oct 4, 2015 11:03 PM
Post by CHARLES AGU on October 4, 2015
Hello, please what would you call the relationship between two superimposable mirror images of structures with 4 different groups each? I'm performing a dry lab using my model kit (the structures were enantiomers at first but when I switched two of the groups on the mirror image, it became identical and superimposable with the original structure). Thanks.
1 answer
Sat Oct 3, 2015 7:36 PM
Post by CHARLES AGU on October 3, 2015
How do I fast forward or go to the subsection I want in the lecture?
1 answer
Wed Aug 26, 2015 10:21 AM
Post by Rafael Mojica on August 25, 2015
Hello,
When you number the carbon chain the place where you select #! is it random or are there any rules involved with the direction of the numbering?
Thanks!
1 answer
Sat Apr 11, 2015 6:01 PM
Post by Jason Smith on April 10, 2015
Just to keep things clear in my mind: chiral molecules are always non-superimposable and achiral molecules are always superimposable, right? Thank you professor.
2 answers
Sun Mar 22, 2015 11:10 PM
Post by Andres Lojano Bermeo on March 21, 2015
Hello Professor, on minute 53:19, you mention that the configuration is (2R,3R) 2-bromo-3chloropentane, shouldn't the 3R be a 3S, since it is going counter clock-wise to the #4 hydrogen? Or is that incorrect?
1 answer
Fri Dec 5, 2014 1:13 AM
Post by Camille Fraser on December 4, 2014
When drawing (S)-2-bromopentane shouldn't it start from the CH3 instead of the CH2?
1 answer
Fri Dec 5, 2014 1:15 AM
Post by Camille Fraser on December 4, 2014
In 33:07 in Nomenclature why is the Y shaped structure considered number 1, how did you get C,C H?
1 answer
Sun Oct 26, 2014 12:51 AM
Post by Foaad Zaid on October 23, 2014
if we had a c=c double bond, and each of the substituents on the carbons were all different groups, would that molecule be chiral? We have no stereocenters, however it extremely unsymmetrical? Thank you.
1 answer
Sun Oct 26, 2014 12:49 AM
Post by Foaad Zaid on October 23, 2014
hello, at 99: 13 you said that 25% and 25% combine to make a racemic mixture. do you mind just explaining that? Thank you.
1 answer
Sun Oct 26, 2014 12:46 AM
Post by Foaad Zaid on October 23, 2014
hello, at 20:05, for the cis-1,3 disubstituted cyclohexane, are you saying that the the molecule itself is achiral? Is the mirror meant to depict the relationship between 2 different molecules or meant to depict the plane of symmetry in just ONE cis- 1,3 disubstituted cyclohexane? Because to me, if we were to treat the molecules on each side of the mirror as separate molecules, they would be enantiomers of eachother correct? Thank you in advance.
1 answer
Mon Oct 20, 2014 11:55 PM
Post by Robert Rakowski on October 20, 2014
Hello Dr. Starkey,
What makes a carbon a chirality center? I understand that it would need to have four different groups but at 18 min in the second example why wouldn't the other carbons be chirality centers? They would have 4 different groups (c,h,h,h) (c,h,h)so why is the carbon with the chlorine a chirality center and the others are not?
1 answer
Tue Oct 14, 2014 12:37 AM
Post by Celeste Shefferly on October 13, 2014
Do you cover polarimetry in any of your lectures?
1 answer
Last reply by: linda chy
Sun Oct 12, 2014 2:36 PM
Post by linda chy on October 12, 2014
you are amazing!!!. i was completely blank when this was being thought in class buy my prof,but it makes so much sense now. Thank you very much:)
1 answer
Sat Sep 27, 2014 2:34 PM
Post by Kevin Golden on September 26, 2014
You are amazing!!!
1 answer
Tue Sep 9, 2014 11:45 PM
Post by Nadia A on September 9, 2014
Hi Educator,
Is there a way to fast forward through lectures, or does the system not allow this? Thx!!
0 answers
Post by Nadia Ariqat on September 9, 2014
In my opinion, stereochem is one of the more confusing topics within organic chemistry (and where a lot of us make mistakes when we're first starting out). Drawing the correct fischer projection has always been a bit challenging for me personally; but when you look at it from the perspective of the stick figure/laying down, it actually makes 100% sense. This is a great lecture..highly recommend it!!
0 answers
Post by somia abdelgawad on May 7, 2014
on 64 min also A and C are diastereomers
1 answer
Sat May 10, 2014 12:05 AM
Post by somia abdelgawad on May 7, 2014
you are amazing
1 answer
Fri Apr 4, 2014 9:27 AM
Post by lakshmi tatineni on April 3, 2014
How do i find the meso compounds in a question like this?
For the six isomers given below:
A. RRRR B. RRSS C. SSRR D. SSSS E. RSSR F. RSRS
a) Identify all enantiomer pairs? A and C , B and C
b) Identify all possible meso compounds ????
c) Identify all diastereomers of RRSS. all except B and C (would u include the mesocompounds too (i was thinking no but i have no clue))
Thank You
1 answer
Tue Apr 1, 2014 10:13 PM
Post by Sola Adeonigbagbe on April 1, 2014
Hi, Professor Starkey. Your lectures are very helpful, and its helping me learn Organic Chemistry in a much easier way. I have my exam in a few days, and this has been helping me not panic too much for the exam.
My question is when doing a Fishcer Projection for a molecule, I'm still a bit confused on how to determine whether a substituent is on my left side or my right side. Is there any particular way to determine it if one does not have the molecular kit to practice with? I know I have to look at each chiral carbon at an angle but it's still hard to know why say, bromide is on my left and chlorine is on my right. Thanks!
1 answer
Sat Mar 22, 2014 12:04 AM
Post by Johnny Green on March 20, 2014
At about the 47 minute mark you checked "R" and "S" configuration of the Fischer projection and the line drawing to see if they matched. I understand that when the lowest priority group is pointing toward you (solid wedge) an S configuration is made an R configuration and vice versa. If the lowest priority group is already facing in the plane (dash line) then an R configuration is an R and an S configuration remains an S. You also said that if you imagined laying down looking up the molecule that Cl would be on the left side and H would be on the right side. Why did you reverse the direction from S to R on the Fischer projection? Isn't H already on a dashed line (facing away from you or facing in the plane)?
1 answer
Wed Mar 12, 2014 10:43 PM
Post by Richard Meador on March 12, 2014
on the Fischer projections (56:31), your stick figure lays down and looks up but how do you know the direction to orient his feet(right or left)?
1 answer
Thu Dec 5, 2013 11:36 PM
Post by bwalya nkonde on December 5, 2013
Hello, for the example about the molecules with 2 chiral centers, I am wondering how it is that you ended up with 3R, because it seems we rotated clockwise and not anti-clockwise?
1 answer
Mon Nov 11, 2013 10:05 AM
Post by Christina Elder on November 9, 2013
Hi Dr. Starkey,
With regard to enantiomers, I am confused about superimposability. From my understanding an enantiomers are molecules that are nonsuperimposable mirror images of each other. However, at minute 40:20 in the lecture, where you draw the enantiomer of the (S) 2-bromopentane by inverting the chiral centers, you mention that it is the same compound since they would line up nicely which is the same as superimposable. Is that correct? If so, why would it be considered an enantiomer if it is superimposable, or are they simply just the same compound. Thanks in advance for your help.
Christina
1 answer
Tue Sep 3, 2013 11:50 PM
Post by Donna maria on September 3, 2013
Sorry one more question. The enantiomer example. Isn't this superimposible? Therefore, can it be optically active? I thought achiral molecules could not or is thhis only for r/s configuration?
1 answer
Fri Aug 30, 2013 8:42 PM
Post by Donna maria on August 29, 2013
sorry. I understood the configuration R/S rule on the projection much better than the line drawing. So if i can convert to projections in exam, i should be fine. thank you.
1 answer
Fri Aug 30, 2013 8:48 PM
Post by Donna maria on August 29, 2013
Thank you, your lectures are amazing. I just have a question. Set A example. When you are outlining the second R configuration, I am confused why it is R? I can see that number 1 priority should be Cl (higher atomic number) but the others not labelled or have been omitted, I am unsure about, could you clarify just for peace of mind please?
And also the location points of the dash lines. What determines the location of a dashed line as i can see that there have been numerous examples of different location points (left to the Br or right to the Cl). They are also mostly represented as hydrogen? Are they a another way of showing the side points on a tetrahedral? In addition, they are opposite to the wedges in terms of R/S configuration-is this a standard rule? So sorry for the questions!
1 answer
Tue Apr 23, 2013 3:54 PM
Post by Alicia DaSilva on April 23, 2013
Here is one of my homework questions: a chiral carbon has the following molecules attached to it:
1. CH(CH3)2
2. CH2CH2Br
3. CH2CH
4.H
Question, why does the CH(CH3)2 has a higher priority than CH2CH2Br?
1 answer
Fri Mar 29, 2013 1:51 AM
Post by ahmed alzeory on March 28, 2013
hello dr
for the nomenclature example the last one with hydrogen and bromide, how is it achiral even though the hydrogen and the bromide do not go within the line of symmetry?
1 answer
Thu Mar 7, 2013 11:11 AM
Post by Kristine Penalosa on March 6, 2013
Dr. Starkey,
Is there any way to put the slides in powerpoint format, so we can print the slides all at once?
Thanks for all the lectures!
1 answer
Sat Jan 26, 2013 9:45 PM
Post by marsha prytz on January 26, 2013
at 29:39 you are numbering the 4 groups attached to the chiral center. I was following until you got to the tie. You stated the end carbon with H,H,H was ranked #3 and the carbon #2 was ranked #2 group of the carbon because it had C,H,H. What I'm confused is why you didn't include the C with the 3 H's? Isn't there a carbon there?
1 answer
Sun Jan 6, 2013 1:21 PM
Post by wow love on January 6, 2013
Hi,
you said same physical property, what about chemical property?
1 answer
Sun Jan 6, 2013 1:20 PM
Post by wow love on January 6, 2013
Hi Dr. Starkey
In the 60 min when you were talking about fischer projection, I am kind of confuse about how to figure when the atom would be on your left or right??? I see the little stick figure you make however, how do one differentiate if a dash or wedge would be the atom on your left or right???
1 answer
Sun Dec 16, 2012 4:39 PM
Post by Susan McConnell on December 16, 2012
Hi Dr Starkey,
are there any lectures on Ramachandran plots and phi and psi angles? thanks
0 answers
Post by Alena Schwartsman on October 25, 2012
Lecture has to download completely to be able to skip around (there is loading bar at the bottom of a lecture video). After launching any lecture just wait for a few minutes and than skip around. Hope this helps.
3 answers
Sat Oct 26, 2013 9:59 PM
Post by Susan Reid on July 20, 2012
can you forward on the lecture?
2 answers
Last reply by: Eduardo Castaneda
Mon Jun 18, 2012 7:52 PM
Post by Eduardo Castaneda on June 15, 2012
Dr. Starkey
On example 4 for Nomenclature the Carbon that had 2 carbons, H, and Br attached to it. did you have to look at the CH3's that were next to the CH2's to determine that they were Identical?
For example, I saw that the Carbon had 4 groups attached to it. Out of these four groups 2 of them were the same the "CH2." I stopped there and determined that it was an achiral carbon. You however went forward and compared the groups next to them. Why did you compare the groups next to the CH2 which were the CH3's. Do you have to go further out?
Because lets say that the group on one of the CH2's had a "Cl" attached and on the other side the CH2 had a "N" attached to it. Would that then make it a chiral center?
Im just confused as to why you went further out and compared the groups that were next to the CH2. This then brings up the questions that I asked.
1 answer
Sun Apr 29, 2012 9:40 AM
Post by michelle daane on April 28, 2012
Dr. Starkey,
I'm confused... How is it that when evaluating a molecule for r/s that you can changed sides of the molecule top/bottom....? If I look at the structure and put 1 up, both br and cl are wedges so would be on the same side...., say the right...
When you have more than one arm on the fischer, do all of the arms come towards you or is it every other one ex; back and forth?
thanks
1 answer
Tue Mar 6, 2012 11:09 AM
Post by Michelle Gavin on March 2, 2012
you need to change the program so that you can move forward and backward within a session so you can navigate directly to the section that is of interest.
1 answer
Sun Nov 20, 2011 9:22 AM
Post by christopher coppins on November 13, 2011
hi Dr.Starkey im taking organic 1 an we are now on test #3 which is covering the topics of stereochemistry, alkyl halides, and reactions of alkyl halides.....i see the stereochemistry lecture but i just wanted to make sure for the alkyl lectures which lecture topic will that fall under, will it be the SN1 & SN2 lectures? an by the way thank you so much your the reason why im passing organic 1....
1 answer
Thu Oct 20, 2011 11:15 PM
Post by Ling Huang on October 10, 2011
This lecture was very helpful. I have some questions that I would like to clarify. I thought that enantiomers is chiral and is non-superimposable but for the example of the (S)-2-bromopentane, the mirror image of the (s)-2-bromopentane is superimposable. If I reverse the structure and put it on top of each other it is the same structure. Also, for nomenclature, does it have to be all different for the for bonds that is attached to the center C? Thank You for your help.
1 answer
Last reply by: David Fan
Thu Mar 14, 2013 1:49 PM
Post by Professor Starkey on February 25, 2011
Occurred to me while pushing into fullscreen mode, I cannot see the teacher in the corner of the screen. Any examples not shown on the writing pad are not seen while in full screen mode. Might be nice to be able to see the professor still while in full screen mode.